- 1Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 2China CDC Key Laboratory of Radiological Protection and Nuclear Emergency, National Institute for Radiological Protection, China Centers for Disease Control, Beijing, China
Vitiligo is a multifactorial reversible skin disorder characterized by distinct white patches that result from melanocyte destruction. Activated CXCR3+ CD8+ T cells promote melanocyte detachment and apoptosis through interferon-gamma (IFN-γ secretion and chemokines secreted by keratinocytes through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT)-1 signaling pathway results in further recruitment of CXCR3+ CD8+ T cells and the formation of a positive-feedback loop. JAK inhibitors target the JAK/STAT pathway and are now approved to treat many immune-related diseases. In the treatment of vitiligo, JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, are effective, supporting the implication of the IFN-γ-chemokine signaling axis in the pathogenesis of vitiligo. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Introduction
Vitiligo is an acquired, idiopathic autoimmune disorder characterized by patchy depigmentation in the skin, hair, or both (1). The disorder affects approximately 0.5–2% of adults and children globally and presents with amelanotic, milky white, and well-demarcated macules or patches surrounded by normal skin (2). Patients with vitiligo, especially those with darker skin tones, can suffer from stigmatization, negatively impacting their mental health and quality of life (3–5). Traditional therapeutic methods include systemic glucocorticoids and phototherapy. Although new therapeutic methods have been tested in clinical trials, universally effective treatment for vitiligo remains elusive because of the incomplete understanding of its pathogenesis (1, 6–8).
The Interferon-Gamma-Chemokine Axis in the Pathogenesis of Vitiligo
Depigmentation that characterizes vitiligo is caused by progressive melanocyte destruction (8). Both in vivo and ex vivo studies have provided strong evidence that melanocyte-specific CD8+ T cells predominantly infiltrate the dermal-epidermal junction adjacent to melanocytes in the border of depigmented lesions and participate in the elimination and destruction of melanocytes (9–12).
IFN-γ the key cytokine produced by CD8+ T cells, plays a central role in the pathogenesis of the disease (13). The expression of IFN-γ-induced genes including the T cell chemokine receptor (CXCR3) and its multiple ligands, CXCL9, CXCL10, and CXCL11, is upregulated in depigmented skin lesions. The expression of IFN-γ-induced genes is consistent with other findings: enriched infiltration of CXCR3+ CD8+ T cells, including melanocyte-specific CD8+ T cells, found in biopsies of vitiligo lesions, and increased CXCR3 receptor expression on melanocyte-specific T cells in the blood and skin of patients with vitiligo (14–18).
Based on multiple studies conducted in mouse vitiligo models, the IFN-γ-chemokine axis, with its associated positive feedback loop, has been identified as a potential pathway in the initiation and progression of vitiligo. Autoreactive CD8+ T cells produce IFN-γ, which promotes depigmentation. IFN-γ simultaneously stimulates keratinocytes to express CXCR3, which binds to CXCL9 to recruit more melanocyte-reactive T cells. In addition, CXCL10 recruits T cells within the skin through the CXCR3 receptor, which prolongs and exacerbates the established vitiligo lesion (Figure 1C) (15, 19–21).
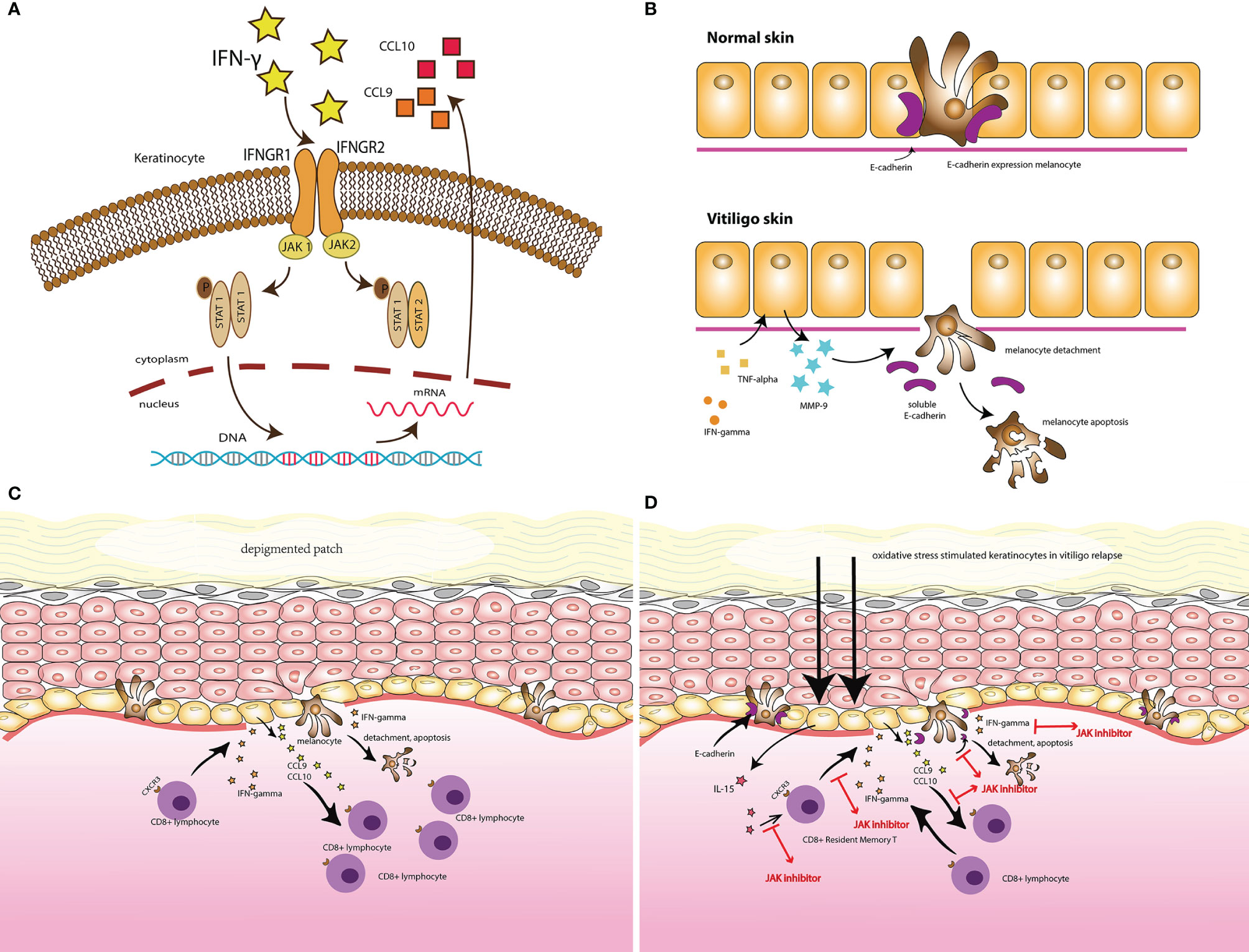
Figure 1 (A) IFN- γ signaling and the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway in vitiligo. (B) The secretion of MMP-9 by keratinocytes, in response to IFN-γ and TNF-α, induced melanocytes detachment through E-cadherin disruption and released its soluble form, in the vitiligo skin comparing with the normal skin. (C) Illustrates the vitiligo pathogenesis: the IFN-γ-chemokine axis, with its associated positive-feedback loop: the autoreactive CD8+ T cells produce IFN-γ, which promotes depigmentation; IFN-γ simultaneously stimulates keratinocytes to express CXCR3 that binds to CXCL9 to recruit more melanocyte-reactive T cells. In addition, CXCL10 recruits T cells within the skin through the CXCR3 receptor, which prolongs and exacerbates the established vitiligo lesion. (D) The potential target for JAK inhibitors to treat vitiligo.
IFN-γ Signaling and the Janus Kinase/Signal Transducer and Activator of Transcription Pathway In Vitiligo
Janus kinases (JAKs) are a family of cytoplasmic tyrosine kinases (TYKs) with a structure consisting of four domains (22). They aid cytokine-mediated signal transduction through the JAK/STAT pathway (23). The key members of this unique tyrosine kinase family include JAK1, JAK2, JAK3, and TYK2 (24). IFNs stimulate the JAK/STAT pathway, leading to the expression of IFN-stimulated genes (ISGs) (25). IFN-γ, a type II interferon, activates the JAK/STAT1 pathway by binding to a cell-surface receptor composed of two subunits, IFNGR1 and IFNGR2, related to JAK1 and JAK2, respectively. This leads to the phosphorylation of STAT1, which initiates gene transcription (26). Given the role of JAK1 and JAK2 in the JAK/STAT pathway, IFN-γ signals can also be blocked by inhibiting JAK1 or JAK2. The JAK/STAT pathway is involved in a variety of immune-related disorders, and inhibitors targeting JAKs are now used to treat numerous diseases (Figure 1A) (27).
Studies have demonstrated the central role of the JAK/STAT pathway in vitiligo. Case-control studies have demonstrated a stepwise pattern of increased expression of JAK1 and JAK3 in lesion-free, perilesional, and vitiliginous skin, respectively, and high expression of JAK 1 was also shown to decline after narrow-band ultraviolet B therapy (23, 28). When the JAK/STAT signaling pathway is inhibited, the detachment of low E-cadherin-expressing melanocytes in the basal layer of the epidermis, a critical step before melanocyte apoptosis, is disrupted. This has also been shown to decrease MMP-9, the key potent factor secreted by keratinocytes in response to IFN-γ and TNF-α, which was found increased in the skin and serum vitiligo patients and stimulated E-cadherin disruption (29, 30). Interleukin (IL)-15 is a cytokine with a special delivery mechanism that transmits signals through the JAK/STAT pathway with JAK1 and JAK3 stimulation, which results in STAT5 activation (31). In the pathogenesis of vitiligo relapse, oxidative stress stimulates keratinocytes via nuclear factor (NF)-κB signaling to express IL-15 and IL-15 Rα, which activate CD8+ resident memory T cells through the JAK/STAT signaling pathway (Figure 1B) (32).
The Use of JAK Inhibitors in Vitiligo Treatment
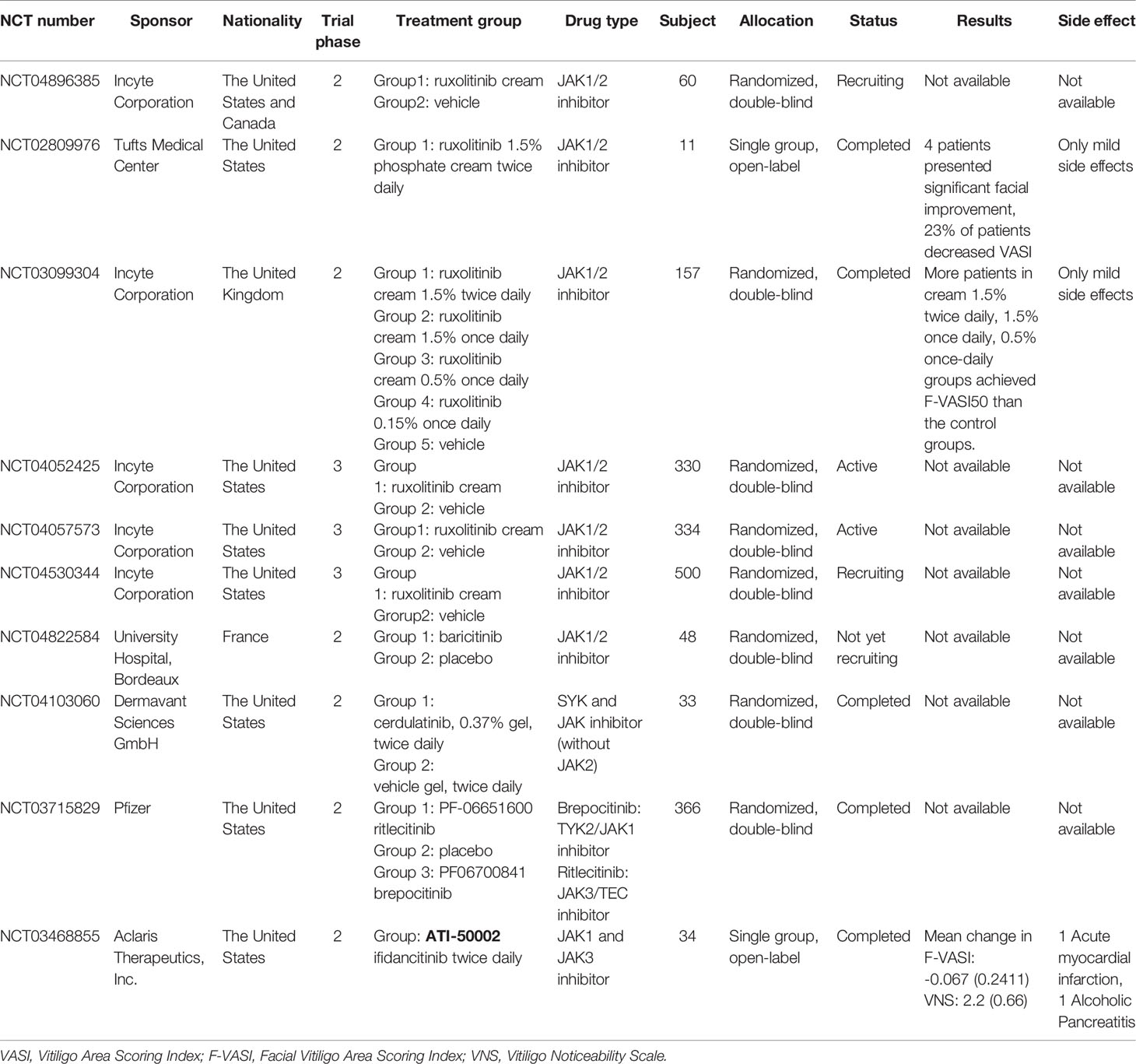
In mouse vitiligo models, neutralization of IFN-γ antibodies prevent the accumulation of CD8+ T cells and lesion depigmentation. JAK inhibitors have been shown to block IFN-γ signaling, contributing to re-pigmentation in individuals with vitiligo. Tofacitinib (Pfizer, New York, NY, USA), ruxolitinib (Celgene, Summit, NJ, USA), and baricitinib (Indianapolis, IN, USA) are the three most commonly reported JAK inhibitors used in vitiligo treatment (Figure 1D) (Tables 1A, B).
Ruxolitinib
Ruxolitinib (INCB-018424) is a small-molecule inhibitor that selectively targets JAK1 and JAK2 (38). The oral form of ruxolitinib was first approved in 2011 to treat polycythemia vera, essential thrombocythemia, and myelofibrosis. Although oral ruxolitinib has been shown to improve skin conditions, such as alopecia areata (39, 40), topical administration of ruxolitinib resulted in higher concentrations in both the epidermis and dermis with minimal deleterious systemic effects versus oral administration, demonstrating sustained and near-complete blockage of the JAK/STAT signaling pathway in the tissues to which it was applied, with negligible plasma concentrations (41). Therefore, more studies have been conducted on ruxolitinib cream to investigate its efficacy in treating inflammatory skin disorders, such as alopecia areata, atopic dermatitis, lichen planus, and psoriasis (41, 42).
Rapid skin re-pigmentation was observed in male patients with vitiligo and alopecia areata treated with oral ruxolitinib, with marked declines in serum CXCL10 levels after administration, indicating that ruxolitinib’s mechanism of action may involve disruption of IFN-γ signaling and JAKs. The role of IFN-γ- and CD8+ T cell-dependent cytokine activity, which is implicated in the pathogenesis of alopecia areata, may thus also play a role in the pathogenesis of vitiligo (15, 43–45). Although the mechanism of action of ruxolitinib cream in the treatment of vitiligo is still unclear, studies on mice and human tissues have found that in addition to blocking IFN-γ and its downstream effector, JAKs, ruxolitinib also inhibited the differentiation and migration of human dendritic cells (DCs) ex vivo. This reduced DC-induced antigen-specific CD4+ and CD8+ T cell responses and the induction of CD8+ cytotoxic T cell responses, which are the key cell responses that are hypothesized to participate in the pathogenesis of vitiligo, in vivo (46). An ongoing phase 2 study (NCT04896385), sponsored by the Incyte Corporation, is currently in the recruitment stage. This study aimed to investigate the mechanism of action of ruxolitinib cream in treating patients with vitiligo by evaluating changes in immune biomarkers, including CXCL10 (47).
Based on preliminary findings in mice and human tissues, clinical trials with topical ruxolitinib have been conducted. In a 20-week, open-label, phase 2 trial (NCT02809976), 12 patients received topical 1.5% ruxolitinib cream applied to vitiligo lesions twice daily. Compared with baseline, four patients showed significant improvement in facial lesions, and all patients showed a 23% average decrease in the Vitiligo Area Scoring Index (VASI) (48). A 32-week extension study followed, but no improvement was found in skin lesions that were previously non-responsive to ruxolitinib. However, five patients followed up after the trial maintained their response to treatment for up to 6 months after treatment discontinuation (49).
In another double-blind phase 2 trial (NCT03099304), 157 adult patients with vitiligo from 26 hospitals in the United States were randomly assigned 1:1:1:1:1 to receive topical ruxolitinib cream 1.5% twice daily, 1.5% once daily, 0.5% once daily, 0.15% once daily, or a vehicle for 24 weeks, respectively. The percentage of patients who achieved more than 50% improvement from baseline in Facial VASI (F-VASI 50) at week 24 was set as the primary endpoint to evaluate treatment efficacy in each group. After the 24-week treatment period, significantly more patients in the groups receiving ruxolitinib cream 1.5% twice daily, 1.5% once daily, and 0.5% once daily achieved F-VASI50 than patients in the control groups. Patients who were assigned in the three positive responsive groups receiving ruxolitinib cream 1.5% twice daily, 1.5% once daily, and 0.5% once daily were asked to remain their original treatment dose up to 52 weeks. At week 52, patients in these three treatment groups showed substantial repigmentation of vitiligo lesions and good dose tolerance, indicating that topical ruxolitinib may be a good option for vitiligo management (50).
The goal of the two phase 2 studies was to assess the generalizability and investigate the efficacy of different concentrations and dosages of ruxolitinib cream (51). However, the average age of patients in both studies was >40 years, and only patients with non-segmental vitiligo were included. There were no studies specified to younger patients between 20 and 30 years of age (the most affected age group), and patients with vitiligo with darker skin (the patient group are more likely to be associated with concurrent autoimmune conditions) (52, 53). In both studies, enrolled patients’ total affected body surface area was under 10% on average, and the topical ruxolitinib exposure was under 3.74 g. Although two multi-center, double-blind, vehicle-controlled phase 3 studies (NCT04052425 and NCT04057573) and one 52-week long-term large sample phase 3 study (NCT04530344) are now being performed to evaluate the efficacy, safety, and the duration of response following ruxolitinib cream withdrawal, further evaluation of the use of ruxolitinib cream is needed to determine its safety and efficacy when used in patients with larger areas of vitiligo lesions (54–57).
Tofacitinib
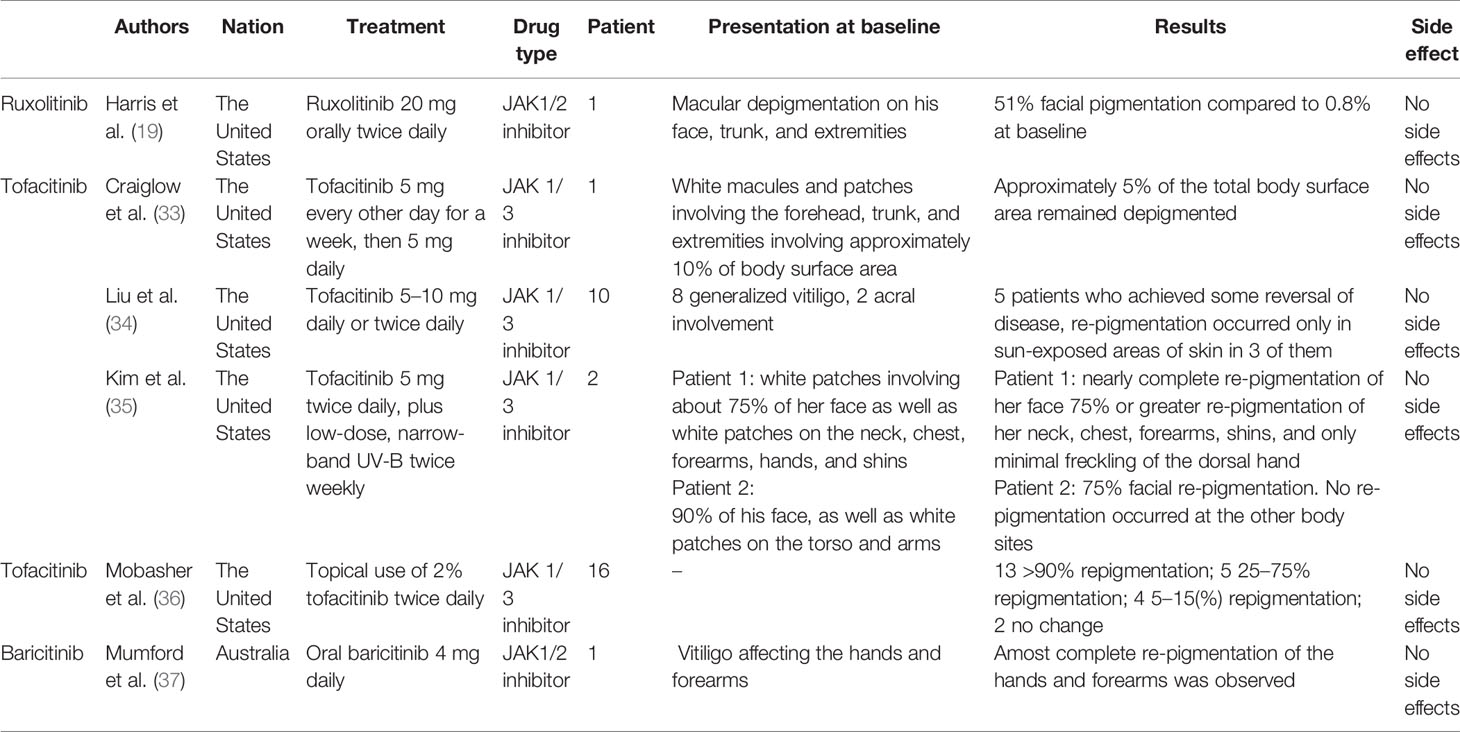
Tofacitinib is a selective JAK1 and JAK3 inhibitor approved to treat moderate and severe rheumatoid arthritis (43). Both oral and topical forms of tofacitinib have shown efficacy in treating immune-mediated skin disorders, including plaque psoriasis, atopic dermatitis, and alopecia areata (58–60). Oral administration of tofacitinib was first used in a female patient with vitiligo who had approximately 10% depigmentation in her total body surface area, which was unresponsive to the traditional application of topical corticosteroid ointment and tacrolimus ointment. Given the hypothesized common pathogenesis in alopecia areata and vitiligo, the patient was prescribed 5 mg of oral tofacitinib citrate on alternate days, which was increased to 5 mg daily from week 4. After 5 months of treatment, only 5% of the patient’s total body surface area remained depigmented. No side effects were reported during the treatment period (33).
In a retrospective study of 10 tofacitinib-treated patients with vitiligo, changes in their autoimmune responses were evaluated through suction blister sampling. Ten patients underwent tofacitinib therapy at a dosage of 5–10 mg daily or twice daily for an average of 9.9 months, with only half of the patients achieving re-pigmentation that occurred in sun-exposed areas or areas that received phototherapy only. Flow cytometry revealed a decline in the number of CD8+T cells after tofacitinib treatment, but there was no change in the percentage of melanocyte-specific T cells. In addition, chemokines, such as CXCL9 and CXCL10, were reduced and became undetectable after tofacitinib treatment (34). These findings indicate that re-pigmentation of vitiligo lesions may require both JAK inhibitors (to inhibit local inflammation) and light exposure (to stimulate melanocytes) (35), which is consistent with the finding that sun-exposed areas, such as the hands and face, are more responsive to topical ruxolitinib treatment (48).
Topical 2% tofacitinib cream was also administered to 16 patients with vitiligo, including 11 patients with generalized vitiligo. Consistent with previous studies, more significant responses were noted for facial lesions and patients with darker skin types, while no superior responses were observed in those who received concomitant phototherapy, which contrasts with previously reported results (36). There are no registered clinical trials currently being performed to investigate the use of tofacitinib in vitiligo treatment, and further research is necessary to determine its safety and efficacy, as well as the role of phototherapy in combination with tofacitinib.
Baricitinib
Only one case report has described re-pigmentation in vitiligo lesions treated with baricitinib, a selective JAK1 and JAK2 inhibitor (43). A 67-year-old man with vitiligo involving the hands and forearms noticed complete re-pigmentation when he substituted his tofacitinib 5 mg twice daily with baricitinib 4 mg daily for the treatment of rheumatoid arthritis (37). A new phase 2 study (NCT04822584) is currently being conducted to evaluate the safety and efficacy of the combination of baricitinib and phototherapy in vitiligo treatment (61).
Other JAK Inhibitors
A few registered ongoing trials are focusing on the use of second-generation JAK inhibitors in the treatment of patients with vitiligo, which may elucidate additional inflammatory pathways that may be important in the pathogenesis of vitiligo (13). Cerdulatinib (PRT062070) is a small molecule reversible inhibitor that targets both spleen tyrosine kinase (SYK) and JAK family members while sparing JAK2 (62, 63). A study (NCT04103060) was registered to assess the safety and tolerability of 0.37% cerudulatinib gel in treating adult patients with vitiligo (64).
Ritlecitinib (PF-06651600), a novel irreversible inhibitor of JAK3 and tyrosine kinase expressed in the hepatocellular carcinoma (TEC) kinase family, was previously used in the treatment of moderate-to-severe rheumatoid arthritis (65). Brepocitinib (PF-06700841) is an oral TYK2/JAK1 inhibitor used to treat moderate-to-severe plaque psoriasis (66). An ongoing registered trial (NCT03715829) carried out by Pfizer compared both ritlecitinib and brepocitinib in vitiligo treatment with/without the combination of phototherapy, which may offer additional insights into vitiligo pathogenesis (1, 67).
Ifidancitinib (ATI-50002) is another dual JAK1, and JAK3 inhibitor used to treat alopecia areata with oral and topical formulations (2, 68). The efficacy of a 0.46% ifidancitinib solution on non-segmental facial vitiligo was investigated through a phase 2, open-label study (NCT03468855). Of the 34 enrolled patients, 23 completed the 24-week treatment protocol with ifidancitinib Solution twice daily and improved F-VASI and the Vitiligo Noticeability Scale (VNS) (2, 69).
Discussion
As more studies have investigated the pathogenesis of vitiligo over the past few decades and discovered more targeted, effective, and promising treatments. With the discovery of the role of the IFN-γ signaling axis in vitiligo, more clinical trials with JAK inhibitors have been performed, demonstrating remarkable efficacy in vitiligo management. However, there may be additional inflammatory pathways involved in the pathogenesis of vitiligo, which are yet to be elucidated. TYK2, another member of the JAK family, plays a ubiquitous role in signal transduction with type I interferon (IFN-α), which also induces the expression of CXCL9 and CXCL10 by keratinocytes in vitiligo (70). The common pathogeneses between vitiligo and other autoimmune diseases may also provide novel insights into vitiligo, such as the discovery of ruxolitinib and baricitinib in vitiligo treatment. Whether phototherapy is required to stimulate melanocyte regeneration in vitiligo treatment remains controversial, and future studies should be conducted to determine the optimal treatment regimen. Finally, current studies rarely focus on generalized vitiligo; hence, research should be conducted in patients who require topical JAK inhibitors to larger body surface areas. The differences in efficacy and safety between oral and tropical formulations of JAK inhibitors are another opportunity for future research.
Author Contributions
FL and LG contributed to the conception and design of this study. FQ wrote the first draft of this manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by Beijing Municipal Natural Science Foundation (7202139).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage for English language editing.
References
1. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet (2015) 386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7
2. Bergqvist C, Ezzedine K. Vitiligo: A Review. Dermatology (2020) 236(6):571–92. doi: 10.1159/000506103
3. Salzes C, Abadie S, Seneschal J, Whitton M, Meurant JM, Jouary T, et al. The Vitiligo Impact Patient Scale (VIPs): Development and Validation of a Vitiligo Burden Assessment Tool. J Invest Dermatol (2016) 136(1):52–8. doi: 10.1038/JID.2015.398
4. Kussainova A, Kassym L, Akhmetova A, Glushkova N, Sabirov U, Adilgozhina S, et al. Vitiligo and Anxiety: A Systematic Review and Meta-Analysis. PloS One (2020) 15(11):e0241445. doi: 10.1371/journal.pone.0241445
5. Lai YC, Yew YW, Kennedy C, Schwartz RA. Vitiligo and Depression: A Systematic Review and Meta-Analysis of Observational Studies. Br J Dermatol (2017) 177(3):708–18. doi: 10.1111/bjd.15199
6. Kubelis-Lopez DE, Zapata-Salazar NA, Said-Fernandez SL, Sanchez-Dominguez CN, Salinas-Santander MA, Martinez-Rodriguez HG, et al. Updates and New Medical Treatments for Vitiligo (Review). Exp Ther Med (2021) 22(2):797. doi: 10.3892/etm.2021.10229
7. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working G. Current and Emerging Treatments for Vitiligo. J Am Acad Dermatol (2017) 77(1):17–29. doi: 10.1016/j.jaad.2016.11.010
8. Migayron L, Boniface K, Seneschal J. Vitiligo, From Physiopathology to Emerging Treatments: A Review. Dermatol Ther (Heidelb) (2020) 10(6):1185–98. doi: 10.1007/s13555-020-00447-y
9. Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, et al. Immunopolarization of CD4+ and CD8+ T Cells to Type-1-Like Is Associated With Melanocyte Loss in Human Vitiligo. Lab Invest (2003) 83(5):683–95. doi: 10.1097/01.lab.0000069521.42488.1b
10. van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune Destruction of Skin Melanocytes by Perilesional T Cells From Vitiligo Patients. J Invest Dermatol (2009) 129(9):2220–32. doi: 10.1038/jid.2009.32
11. Strassner JP, Rashighi M, Ahmed Refat M, Richmond JM, Harris JE. Suction Blistering the Lesional Skin of Vitiligo Patients Reveals Useful Biomarkers of Disease Activity. J Am Acad Dermatol (2017) 76(5):847–55 e5. doi: 10.1016/j.jaad.2016.12.021
12. Palermo B, Campanelli R, Garbelli S, Mantovani S, Lantelme E, Brazzelli V, et al. Specific Cytotoxic T Lymphocyte Responses Against Melan-A/MART1, Tyrosinase and Gp100 in Vitiligo by the Use of Major Histocompatibility Complex/Peptide Tetramers: The Role of Cellular Immunity in the Etiopathogenesis of Vitiligo. J Invest Dermatol (2001) 117(2):326–32. doi: 10.1046/j.1523-1747.2001.01408.x
13. Frisoli ML, Essien K, Harris JE. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu Rev Immunol (2020) 38:621–48. doi: 10.1146/annurev-immunol-100919-023531
14. Grimes PE, Morris R, Avaniss-Aghajani E, Soriano T, Meraz M, Metzger A. Topical Tacrolimus Therapy for Vitiligo: Therapeutic Responses and Skin Messenger RNA Expression of Proinflammatory Cytokines. J Am Acad Dermatol (2004) 51(1):52–61. doi: 10.1016/j.jaad.2003.12.031
15. Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Sci Transl Med (2014) 6(223):223ra23. doi: 10.1126/scitranslmed.3007811
16. Wang XX, Wang QQ, Wu JQ, Jiang M, Chen L, Zhang CF, et al. Increased Expression of CXCR3 and Its Ligands in Patients With Vitiligo and CXCL10 as a Potential Clinical Marker for Vitiligo. Br J Dermatol (2016) 174(6):1318–26. doi: 10.1111/bjd.14416
17. Boniface K, Jacquemin C, Darrigade AS, Dessarthe B, Martins C, Boukhedouni N, et al. Vitiligo Skin Is Imprinted With Resident Memory CD8 T Cells Expressing Cxcr3. J Invest Dermatol (2018) 138(2):355–64. doi: 10.1016/j.jid.2017.08.038
18. Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K, et al. Type I Interferon Signature in the Initiation of the Immune Response in Vitiligo. Pigment Cell Melanoma Res (2014) 27(3):398–407. doi: 10.1111/pcmr.12219
19. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A Mouse Model of Vitiligo With Focused Epidermal Depigmentation Requires IFN-Gamma for Autoreactive CD8(+) T-Cell Accumulation in the Skin. J Invest Dermatol (2012) 132(7):1869–76. doi: 10.1038/jid.2011.463
20. Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, et al. Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis During Vitiligo and May Serve as Biomarkers of Disease. J Invest Dermatol (2017) 137(2):350–8. doi: 10.1016/j.jid.2016.09.016
21. Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, Harris JE. CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. J Invest Dermatol (2017) 137(4):982–5. doi: 10.1016/j.jid.2016.10.048
22. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs (2017) 77(5):521–46. doi: 10.1007/s40265-017-0701-9
23. Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus Kinase 1 in Vitiligo & Psoriasis Before and After Narrow Band UVB: A Case-Control Study. Arch Dermatol Res (2018) 310(1):39–46. doi: 10.1007/s00403-017-1792-6
24. Rane SG, Reddy EP. Janus Kinases: Components of Multiple Signaling Pathways. Oncogene (2000) 19(49):5662–79. doi: 10.1038/sj.onc.1203925
25. Nan Y, Wu C, Zhang YJ. Interplay Between Janus Kinase/Signal Transducer and Activator of Transcription Signaling Activated by Type I Interferons and Viral Antagonism. Front Immunol (2017) 8:1758. doi: 10.3389/fimmu.2017.01758
26. Platanias LC. Mechanisms of Type-I- and Type-II-Interferon-Mediated Signalling. Nat Rev Immunol (2005) 5(5):375–86. doi: 10.1038/nri1604
27. O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu Rev Med (2015) 66:311–28. doi: 10.1146/annurev-med-051113-024537
28. Abdel Motaleb AA, Tawfik YM, El-Mokhtar MA, Elkady S, El-Gazzar AF, ElSayed SK, et al. Cutaneous JAK Expression in Vitiligo. J Cutan Med Surg (2021) 25(2):157–62. doi: 10.1177/1203475420972340
29. Boukhedouni N, Martins C, Darrigade AS, Drullion C, Rambert J, Barrault C, et al. Type-1 Cytokines Regulate MMP-9 Production and E-Cadherin Disruption to Promote Melanocyte Loss in Vitiligo. JCI Insight (2020) 5(11):e133772. doi: 10.1172/jci.insight.133772
30. Khokha R, Murthy A, Weiss A. Metalloproteinases and Their Natural Inhibitors in Inflammation and Immunity. Nat Rev Immunol (2013) 13(9):649–65. doi: 10.1038/nri3499
31. Stonier SW, Schluns KS. Trans-Presentation: A Novel Mechanism Regulating IL-15 Delivery and Responses. Immunol Lett (2010) 127(2):85–92. doi: 10.1016/j.imlet.2009.09.009
32. Chen X, Guo W, Chang Y, Chen J, Kang P, Yi X, et al. Oxidative Stress-Induced IL-15 Trans-Presentation in Keratinocytes Contributes to CD8(+) T Cells Activation via JAK-STAT Pathway in Vitiligo. Free Radic Biol Med (2019) 139:80–91. doi: 10.1016/j.freeradbiomed.2019.05.011
33. Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol (2015) 151(10):1110–2. doi: 10.1001/jamadermatol.2015.1520
34. Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in Vitiligo Using the Janus Kinase Inhibitor Tofacitinib May Require Concomitant Light Exposure. J Am Acad Dermatol (2017) 77(4):675–82 e1. doi: 10.1016/j.jaad.2017.05.043
35. Kim SR, Heaton H, Liu LY, King BA. Rapid Repigmentation of Vitiligo Using Tofacitinib Plus Low-Dose, Narrowband UV-B Phototherapy. JAMA Dermatol (2018) 154(3):370–1. doi: 10.1001/jamadermatol.2017.5778
36. Mobasher P, Guerra R, Li SJ, Frangos J, Ganesan AK, Huang V. Open-Label Pilot Study of Tofacitinib 2% for the Treatment of Refractory Vitiligo. Br J Dermatol (2020) 182(4):1047–9. doi: 10.1111/bjd.18606
37. Mumford BP, Gibson A, Chong AH. Repigmentation of Vitiligo With Oral Baricitinib. Australas J Dermatol (2020) 61(4):374–6. doi: 10.1111/ajd.13348
38. Mesa RA. Ruxolitinib, a Selective JAK1 and JAK2 Inhibitor for the Treatment of Myeloproliferative Neoplasms and Psoriasis. IDrugs (2010) 13(6):394–403.
39. Almutairi N, Nour TM, Hussain NH. Janus Kinase Inhibitors for the Treatment of Severe Alopecia Areata: An Open-Label Comparative Study. Dermatology (2019) 235(2):130–6. doi: 10.1159/000494613
40. Mackay-Wiggan J, Jabbari A, Nguyen N, Cerise JE, Clark C, Ulerio G, et al. Oral Ruxolitinib Induces Hair Regrowth in Patients With Moderate-to-Severe Alopecia Areata. JCI Insight (2016) 1(15):e89790. doi: 10.1172/jci.insight.89790
41. Persaud I, Diamond S, Pan R, Burke K, Harris J, Conlin M, et al. Plasma Pharmacokinetics and Distribution of Ruxolitinib Into Skin Following Oral and Topical Administration in Minipigs. Int J Pharm (2020) 590:119889. doi: 10.1016/j.ijpharm.2020.119889
42. Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat Rev Drug Discovery (2012) 11(2):103–4. doi: 10.1038/nrd3652
43. McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and Future Status of JAK Inhibitors. Lancet (2021) 398(10302):803–16. doi: 10.1016/S0140-6736(21)00438-4
44. Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid Skin Repigmentation on Oral Ruxolitinib in a Patient With Coexistent Vitiligo and Alopecia Areata (AA). J Am Acad Dermatol (2016) 74(2):370–1. doi: 10.1016/j.jaad.2015.09.073
45. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia Areata Is Driven by Cytotoxic T Lymphocytes and Is Reversed by JAK Inhibition. Nat Med (2014) 20(9):1043–9. doi: 10.1038/nm.3645
46. Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-Inhibitor Ruxolitinib Impairs Dendritic Cell Function In Vitro and In Vivo. Blood (2013) 122(7):1192–202. doi: 10.1182/blood-2013-03-484642
47. Incyte C. A Study to Evaluate the Mechanism of Action of Ruxolitinib Cream in Subjects With Vitiligo (TRuE-V MOA). (2022).
48. Rothstein B, Joshipura D, Saraiya A, Abdat R, Ashkar H, Turkowski Y, et al. Treatment of Vitiligo With the Topical Janus Kinase Inhibitor Ruxolitinib. J Am Acad Dermatol (2017) 76(6):1054–60 e1. doi: 10.1016/j.jaad.2017.02.049
49. Joshipura D, Alomran A, Zancanaro P, Rosmarin D. Treatment of Vitiligo With the Topical Janus Kinase Inhibitor Ruxolitinib: A 32-Week Open-Label Extension Study With Optional Narrow-Band Ultraviolet B. J Am Acad Dermatol (2018) 78(6):1205–7.e1. doi: 10.1016/j.jaad.2018.02.023
50. Rosmarin D, Pandya AG, Lebwohl M, Grimes P, Hamzavi I, Gottlieb AB, et al. Ruxolitinib Cream for Treatment of Vitiligo: A Randomised, Controlled, Phase 2 Trial. Lancet (2020) 396(10244):110–20. doi: 10.1016/S0140-6736(20)30609-7
51. Rosmarin D, Butler K, Kuo F, Harris JE. Ruxolitinib Cream for the Treatment of Vitiligo - Authors' Reply. Lancet (2020) 396(10264):1736. doi: 10.1016/S0140-6736(20)32470-3
52. Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: A Comprehensive Overview Part I. Introduction, Epidemiology, Quality of Life, Diagnosis, Differential Diagnosis, Associations, Histopathology, Etiology, and Work-Up. J Am Acad Dermatol (2011) 65(3):473–91. doi: 10.1016/j.jaad.2010.11.061
53. Dahir AM, Thomsen SF. Comorbidities in Vitiligo: Comprehensive Review. Int J Dermatol (2018) 57(10):1157–64. doi: 10.1111/ijd.14055
54. Uppal SK, Kearns DG, Chat VS, Wu JJ. Ruxolitinib Cream for the Treatment of Vitiligo. Lancet (2020) 396(10264):1735–6. doi: 10.1016/S0140-6736(20)32469-7
57. Incyte C. Assess the Long Term Efficacy and Safety of Ruxolitinib Cream in Participants With Vitiligo. (2024).
58. Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or Adalimumab Versus Placebo for Psoriatic Arthritis. N Engl J Med (2017) 377(16):1537–50. doi: 10.1056/NEJMoa1615975
59. Ibrahim O, Bayart CB, Hogan S, Piliang M, Bergfeld WF. Treatment of Alopecia Areata With Tofacitinib. JAMA Dermatol (2017) 153(6):600–2. doi: 10.1001/jamadermatol.2017.0001
60. Zhou S, Qi F, Gong Y, Zhang J, Zhu B. Biological Therapies for Atopic Dermatitis: A Systematic Review. Dermatology (2021) 237(4):542–52. doi: 10.1159/000514535
61. University Hospital B. Evaluation of Effect and Tolerance of the Association of Baricitinib and Phototherapy Versus Phototherapy in Adults With Progressive Vitiligo. (2023).
62. Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker DC, et al. The Novel Kinase Inhibitor PRT062070 (Cerdulatinib) Demonstrates Efficacy in Models of Autoimmunity and B-Cell Cancer. J Pharmacol Exp Ther (2014) 351(3):538–48. doi: 10.1124/jpet.114.218164
63. Coffey GP, Feng J, Betz A, Pandey A, Birrell M, Leeds JM, et al. Cerdulatinib Pharmacodynamics and Relationships to Tumor Response Following Oral Dosing in Patients With Relapsed/Refractory B-Cell Malignancies. Clin Cancer Res (2019) 25(4):1174–84. doi: 10.1158/1078-0432.CCR-18-1047
64. Dermavant Sciences Gmb H. Safety and Tolerability Study of Cerdulatinib Gel, 0.37% in Adults With Vitiligo. (2020).
65. Robinson MF, Damjanov N, Stamenkovic B, Radunovic G, Kivitz A, Cox L, et al. Efficacy and Safety of PF-06651600 (Ritlecitinib), a Novel JAK3/TEC Inhibitor, in Patients With Moderate-To-Severe Rheumatoid Arthritis and an Inadequate Response to Methotrexate. Arthritis Rheumatol (2020) 72(10):1621–31. doi: 10.1002/art.41316
66. Forman SB, Pariser DM, Poulin Y, Vincent MS, Gilbert SA, Kieras EM, et al. TYK2/JAK1 Inhibitor PF-06700841 in Patients With Plaque Psoriasis: Phase IIa, Randomized, Double-Blind, Placebo-Controlled Trial. J Invest Dermatol (2020) 140(12):2359–70 e5. doi: 10.1016/j.jid.2020.03.962
67. A Phase 2b Study To Evaluate The Efficacy And Safety Profile Of PF-06651600 And PF-06700841 In Active Non-Segmental Vitiligo Subjects.
Keywords: vitiligo, JAK/STAT-1 signaling pathway, JAK inhibitors, chemokines, IFN - interferon
Citation: Qi F, Liu F and Gao L (2021) Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 12:790125. doi: 10.3389/fimmu.2021.790125
Received: 06 October 2021; Accepted: 02 November 2021;
Published: 18 November 2021.
Edited by:
Zuben E. Sauna, United States Food and Drug Administration, United StatesReviewed by:
Christopher Richardson, University of Rochester, United StatesHelen He, Mount Sinai Hospital, United States
Copyright © 2021 Qi, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, roseliufang@qq.com
 Fei Qi
Fei Qi Fang Liu
Fang Liu Ling Gao2
Ling Gao2